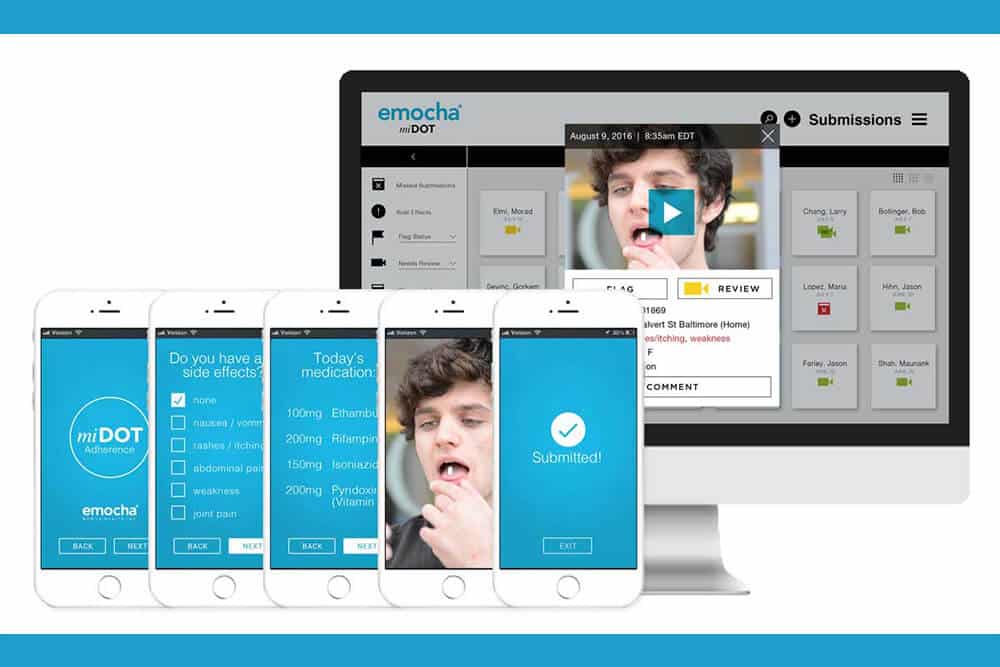
A startup company based in Baltimore has recently been awarded research funding by the National Institute on Drug Abuse (NIDA), through a Small Business Innovation grant. emocha Mobile Health, a company focused on medication adherence, will receive $225,000 through the NIH Fast-Track mechanism, with an additional $1.5 million tied to achieving specific milestones.
Healthcare practitioners and professionals across the United States have witnessed a rapid increase in the use of prescription and non-prescription opioids in the last decade; as a result, drug overdoses are now the leading cause of death among Americans under 50. According to the Drug Enforcement Administration, “Overdose deaths, particularly from prescription drugs and heroin, have reached epidemic levels.” Buprenorphine maintenance therapy is now a frequently utilized treatment option: using a partial agonist opioid to treat acute opioid addiction.
emocha’s project is engineered to demonstrate the viability of ‘video directly observed therapy’ for patients receiving buprenorphine therapy in office-based opioid treatment programs (OBOT). Outlined outcomes of interest include patient adherence, care retention, medication diversion, and measures of illicit opioid use and abstinence.
The practice of directly observed therapy (DOT)—watching a patient ingest each and every dose of medication—is the currently practiced global standard of care in tuberculosis and methadone maintenance. The video-based technology utilized by emocha ensures that DOT is more cost-effective, and scalable. The company has also deployed the technology for hepatitis C and tuberculosis monitoring. A study conducted at Johns Hopkins recently revealed that emocha’s tuberculosis platform ensured that patients achieved a 92% rate, on average, of adherence to their medication regimen, saving providers over $1,400 in monitoring costs per patient.
Extensive literature demonstrates that prescribers of buprenorphine for opioid and substance use disorders experience consistent barriers to treatment expansion through medication diversion and patient non-adherence. Statistics demonstrate that approximately 50% of patients treated with buprenorphine are not retained through maintenance treatment. These patients, as well as those undergoing methadone treatment—which requires DOT—will likely experience better retention and overall outcomes with video directly observed therapy.
emocha will focus on developing the technology for OBOT in collaboration with the University of Washington School of Medicine and Boston Medical Center, two of the leading academic medical centers for substance abuse disorder research. Dr. Judith Tsui, an associate professor of medicine at the UW School of Medicine, remarked: “I am excited for this opportunity to partner with emocha to learn if this new technology can support patients who are engaging in treatment for opioid use disorders with buprenorphine.”
“This project with emocha will allow us as clinicians and researchers to examine a new platform that has potential to expand our medicine bag with a technological aid that helps patients achieve a successful recovery,” said Dr. Jeffrey Samet, Chief of General Internal Medicine at Boston Medical Center.